Active Research Programs in the Chemistry Department
The Chemistry Department has a strong history of conducting research with undergraduates. Our faculty enjoy working and mentoring our students in their labs and exploring the wide field of chemistry together. Below you can find who is actively conducting research in the department. Please reach out to any of the faculty whose research interests you to find out more about our projects and any openings we have in our labs. We look forward to hearing from you!
Douglas Beussman
Anna Brezny
In the Brezny lab, we use electrochemistry to selectively add and remove electrons from organic molecules. This approach enables challenging redox reactions, the construction of complex molecules, and the conversion of simple building blocks into value-added chemicals. Importantly, electrochemical methods provide many benefits over traditional reagent-based redox reactions, such as increased functional group tolerance, milder conditions, fewer safety concerns, and greater sustainability. Our research uses soluble molecular electrocatalysts and electron-proton transfer mediators to control electrocatalytic reactions. Furthermore, by exploring the mechanisms of these reactions, we use our insight and understanding to improve the systems and develop new methods for organic redox chemistry. Our current efforts focus on controlling oxygen atom transfer reactions from O2 to simple alkenes to generate carbonyl compounds and epoxides.
Experimental techniques you will acquire: NMR analysis of complex mixtures, 2D-NMR, electrochemical techniques such as cyclic voltammetry and bulk electrolysis, and column chromatography for purification.
Lab website: https://pages.stolaf.edu/breznylab/
Peter Gittins
My research group is developing alternatives to traditional composite materials using sustainable sources that can be recycled after use to recover starting materials in a circular process. We call these Sustainably Manufactured Advanced Reinforced Thermoplastic (SMART) composites. Students in the SMART Composites Lab use chemistry and engineering to prepare functionalized reinforcing materials, recyclable polymers and model manufacturing systems, working with industry partners and international collaborators to transfer lab-scale chemistry to a production environment. Current projects include: Synthesis of graphene nano-reinforcing agents, circular polymerization of nylon, monitoring polymerization using dielectric analysis, and lab-scale manufacture of composite materials.
Experimental Techniques: Students in the SMART Lab develop techniques in polymer and materials synthesis; various spectroscopic methods including Nuclear Magnetic Resonance (NMR), Infrared (IR), Raman, UV-Vis; Scanning Electron Microscopy (SEM); Elemental Analysis. Students working on engineering projects gain experience building using various fabrication methods including machining and 3-D printing, as well as coding, and 3D design tools.
Paul Jackson

My team works to understand and practice different ways to interrogate and positively impact environmental systems. First, we seek to consider environmental and sustainability footprints associated with operations throughout higher education as well as within the broader chemical sciences. Much of the work this year revolves around the St. Olaf Strategic Plan sustainability objectives related to the UN Sustainable Development Goals, how the campus community is responding, and what strategies should be employed moving forward. In addition to the footprints, my team has an interest in the quality of surface freshwater. Most recently we have worked to ascertain the abundance, distribution and sources of microplastics located in rural, agricultural watersheds, such as rivers and streams within the Cannon River watershed of southeastern Minnesota. Since the boom in synthetic polymer design and manufacturing, plastic has been integrated into all facets of daily life because of its versatility and durability. Unfortunately, that durability generated problems associated with disposal and waste diversion. Processes such as photofragmentation, mechanical forces, and thermal stresses, break plastics down into smaller pieces, becoming microplastics when their size falls below 5 millimeters. In other cases, microplastics were intentionally added to consumer goods to improve performance or contribute to specific functions. Today, microplastics exist as a contaminant in almost every environmental compartment and food web. In addition to the negative health responses associated with their size, microplastics can carry toxic chemicals on their surfaces, causing additional issues when ingested or inhaled. We look to contribute insights into sampling for microplastics as well as add to information associated with their distribution across less populated landscapes.
Experimental techniques you will acquire: environmental sampling, field safety/field work, chemical/laboratory safety, iron catalyzed decomposition, size sorting with mesh sieves, density separations, filtration, use of blanks/controls, data organization, data analysis, life cycle thinking, green chemistry, mass balances, sustainability metrics. Instrumentation includes: ATR-FT-IR spectroscopy, Raman spectroscopy, light microscopy, and scanning electron microscopy.
Lab website: https://pages.stolaf.edu/jackson/project/
Cassie Joiner
The addition of O-linked N-acetylglucosamine (O-GlcNAc) to nuclear and cytoplasmic proteins is essential for cell function and development in eukaryotes. Misregulation of cellular protein O-GlcNAc levels in humans has been implicated in cancers and neurodegenerative diseases. This post-translational modification is catalyzed by a single, nutrient- and stress-sensing enzyme called O-GlcNAc transferase (OGT). OGT is structurally conserved across eukaryotic species, from humans to plants, and modifies over a thousand proteins in almost all cellular processes, including transcription, metabolism, cell signaling, and RNA splicing. Despite its conservation, OGT will target different protein substrates with different sugar-modifications depending on the species being studied. Due to the functional diversity of this structurally conserved protein, the mechanism by which OGT homologs select its substrates remains unclear. The Joiner Lab uses biochemical, chemical biology, and molecular biology techniques to determine how protein structure and protein-protein interactions control nucleotide-sugar and protein substrate selectivity of OGT and its homologs. Current projects in my laboratory aim to (1) understand how OGT interacting partners regulate catalytic activity and protein substrate selection and (2) develop chemical and enzymatic labeling tools to study nuclear and cytoplasmic O-glycosylation.
Experimental techniques you will acquire: bacterial cell culture, recombinant protein expression, protein purification, unnatural amino acid incorporation, photocrosslinking, chemical kinetics and glycosyltransferase assays, SDS-PAGE gel and western blot analysis.
Lab website: https://pages.stolaf.edu/joinerlab/
Laura Listenberger

Excess lipid accumulation contributes to serious medical conditions including heart disease, type II diabetes, and stroke. Surplus lipid is stored inside cells in structures called lipid droplets. All eukaryotic cells, and some bacteria, form lipid droplets. However the biochemical pathways that grow and degrade these organelles are incompletely understood. My laboratory utilizes the model organism Tetrahymena thermophila to identify conserved proteins and biochemical pathways that control lipid droplet synthesis and degradation. We use a combination of computational and biochemical approaches to explore the signals that trigger lipid droplet formation, the enzymes that catalyze lipid breakdown, and the necessity of these pathways for cell survival.
Research skills you will acquire: Students in my laboratory practice reading the scientific literature, experimental design, data analysis, and communication of research results in an environment that emphasizes strong effort and collaboration. Our experiments utilize a variety of research methods such as protein structure analysis, protein purification, enzyme activity assays, thin layer chromatography, fluorescent microscopy, ultracentrifugation, SDS-PAGE and western blot analysis.
Lab website: https://pages.stolaf.edu/listenbergerlab/
Elodie Marlier
In the Marlier lab, we are builders! We aim to study low-valent first-row transition metals complexes by building new hybrid N2P2 ligands. Currently, most industrial processes use expensive second and third row transition metals as catalysts for a variety of transformations. Our group aims to explore cheaper and more widely available first-row transition metals as replacements for these expensive metals. We have created a family of N2P2 ligands to explore the reactivity of low-oxidation state first-row transition metals.
Experimental techniques you will acquire: organic and inorganic synthesis, NMR analysis including 1H, 13C, 31P and 2D-NMR, mass spectrometry, X-ray crystallography.
Jeff Schwinefus
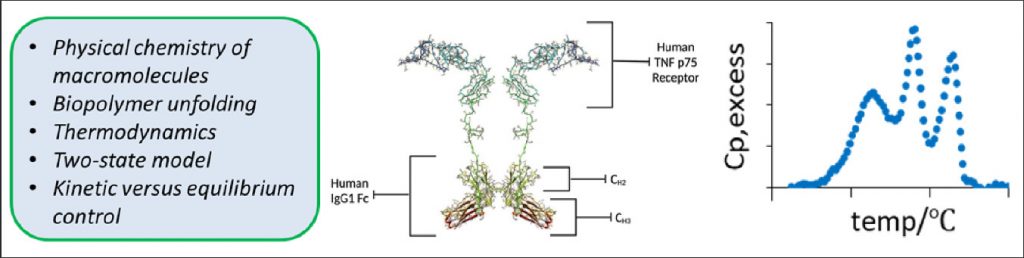
Our research focuses on two projects: 1) We use differential scanning calorimetry (DSC) to measure the thermodynamic and kinetic control for unfolding therapeutic proteins such as etanercept (Enbrel®) and infliximab (Remicade®) to assess optimal formulation conditions and protein lifetimes. 2) We quantify the interaction of neutral organic molecules like urea or amino acids (which we generically call cosolutes) with the chemical surface area of nucleic acids to develop these cosolutes as probes of biopolymer surface area changes. Our goal is to use the attenuation or enhancement in the reaction rate or equilibrium constant to determine the chemical composition and magnitude of surface area changes during DNA, RNA, and protein biochemical reactions.
Research skills you will acquire: Students in our lab gain experience reading articles in the primary literature, preparing solutions, using uv-absorbance spectroscopy and DSC, using Excel and Mathematica, and honing critical thinking and problem-solving skills.
Becca Thompson

Students in the Thompson group study molecules that self-assemble (i.e. spontaneously organize in a single layer) on solid interfaces. These “functionalized surfaces” can be used to create biomedical devices and other interesting materials, but only if we understand the impact that molecular-level disorder can have on the organization of and sensitivity to potential adsorbates, and stability under environmental conditions. We use a specialized form of infrared spectroscopy to observe these molecular assemblies in real time. There are three core projects: 1) Using mixed monolayers (SAMs) to create tunable biosensors; we are trying to determine what factors impact the molecular-level organization of the SAM and its performance at capturing simple blood proteins from solution. 2) Exploring the attachment mechanism and catalytic activity of Cu(II) complexes on a SAM-covered surface. 3) Using SAMs as a model system to understand the (problematic) aqueous aggregation of large aromatic and aliphatic compounds in crude oil.
Research skills you will acquire: Reflection infrared spectroscopy, quartz crystal microbalance, and other surface characterization techniques as needed (microscopy, XRD, contact angle, etc.). Experimental design and instrument development (optics, computational modeling, design and engineering of new parts, etc.).
Learn more: http://pages.stolaf.edu/thompson-lab
You must be logged in to post a comment.